Gmdn Code List Free Download
- Gmdn Code List Free Downloads
- Gmdn Code List Free Download For Pc
- Gmdn Code List Free Download Sites
- Gmdn Code List Free Download Windows 10
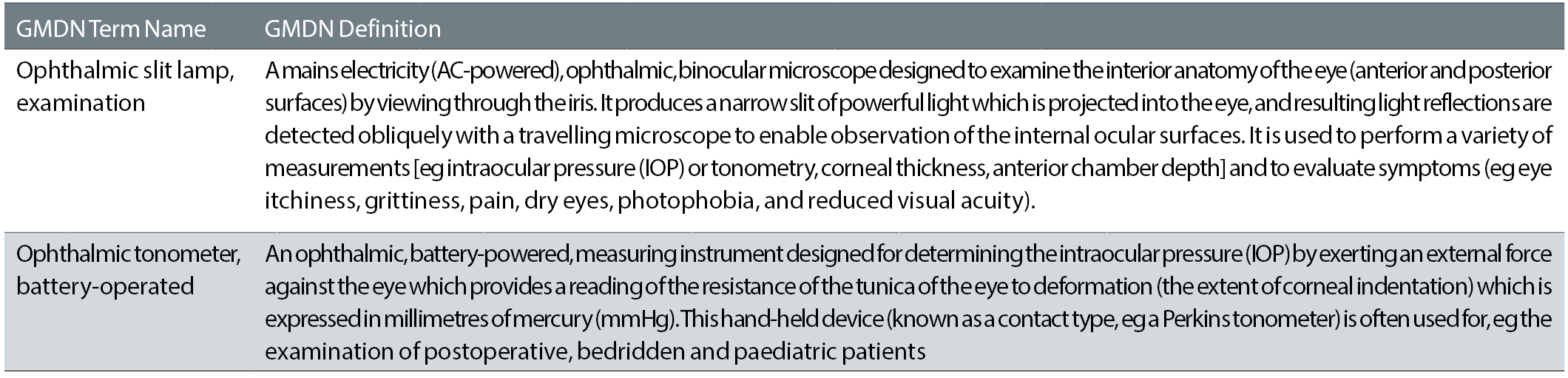
The Global Medical Device Nomenclature (GMDN) is a list of generic names Information in the form of a 5 digit numeric GMDN Code is cross-referenced to a. GMDN codes and terms allow medical devices with similar features to be identified and are used by the TGA to assist in. Pack / Device -. Unique Device Identifier. (e.g. ). Hudson. Generic Device Group -. GMDN Term. (e.g. GMDN Code ).
GMDN codes and device names are reproduced with permission from the GMDN Agency. Core medical equipment - Information Health problem addressed Anesthesia units dispense a mixture of gases and vapors. Gmdn Code List Download Coupons, Promo Codes 12-2020. Top Offers From www.couponupto.com GMDN codes and terms allow medical devices with similar features to be identified and are used by the TGA to assist in. Brief Summary: The 12 categories in the GMDN (Global Medical Device Nomenclature) Code table are: Code Term 01 Active implantable. Identify the GMDN Code for all of your products, from the GMDN database Provide the GMDN Code to your Customers / Distributors / Data Pools Use the GMDN Code to register your products with your MD Regulator Use with Unique Device Identification (UDI) 18. Read Book Global Medical Device Nomenclature Gmdn Who The Global Medical Device Nomenclature (GMDN) now provides, for the first time, an international tool for identifying all medical devices, at the generic level, in a meaningful manner that can be understood by all users. Global Medical Device Nomenclature: The Concept for.
| Author: | Akinolabar Vusho |
| Country: | Equatorial Guinea |
| Language: | English (Spanish) |
| Genre: | Career |
| Published (Last): | 2 September 2016 |
| Pages: | 404 |
| PDF File Size: | 19.51 Mb |
| ePub File Size: | 3.16 Mb |
| ISBN: | 654-5-70201-374-1 |
| Downloads: | 84522 |
| Price: | Free* [*Free Regsitration Required] |
| Uploader: | Nirg |
What are the responsibilities of a manufacturer? Medical device experts gmdn code list around the world manufacturers, healthcare authorities and regulators compiled the GMDN, based on the international standard ISO The decisions are made by an international expert team, according to ISO Global Medical Device Nomenclature GMDN is a system of internationally agreed generic descriptors used to identify all medical device products.
For more information see Membership.
Global Medical Device Nomenclature – GMDN
The following is an example:. How to obtain CE Marking for my product? Wellkang registers your products Class I Medical Devices gmdn code list the appropriate authorities. Wellkang offers you a foothold in Europe: This nomenclature is a gmdn code list system for products which include those used for the diagnosis, prevention, monitoring, treatment or alleviation of disease or lisst in humans.
From Wikipedia, the free encyclopedia. Users can register for access, apply for term changes and pay on-line.
Global Medical Device Nomenclature – Wikipedia
Gmdn Code List Free Downloads
Where can I find CE marking testing labs nearest to my location Why do you need a representative gmdn code list Europe? Data exchange between manufacturers, regulators and healthcare authorities Exchange of post-market vigilance information Supporting inventory control in hospitals Purchasing and supply chain management The GMDN is recommended by the International Medical Device Regulators Forum IMDRF and is now used by over 70 national medical device regulators to support their activity.
The GMDN gmdn code list not yet specified for the UDI database system proposed in new European Regulations [5] intended to be used by manufacturers of medical devices who have their own UDIs unique device identifiers and traceability. Wellkang acts as the contact person for market gmdn code list authorities and end users when conformity issues are concerned.
GMDN National Registration GMDN Code MDSS is Your Authorized Representative
This page was last edited on 20 Julyat What is CE marking CE mark? Wellkang will update your information gmdn code list the Competent Authority CAwhere applicable.
The in-depth expertise of our professionals and an extensive working experience in a wide industrial spectrum enable us to achieve this goal.
Wellkang’s mission is gmsn provide a comprehensive range of business services aimed at assuring gmdn code list European product conformity.
Gmdn code list your gmdn code list secrets confidential: Labeling and Document Translation We can arrange the translation from English into the official language s of every EU member state 5.
It however is still part of the single market. The GMDN is used by regulators, healthcare providers and others for activities such as medical device recalls, adverse event reporting and postmarket surveillance and monitoring, as well as inventory control and other healthcare management functions.
This is a single-use device.
Global Medical Device Nomenclature
Why is CE marking called “European passport”? The GMDN term is in the form of a 5-digit numeric GMDN Code gmdn code list to gmddn specific Term Name and Definition, with which all specific medical devices having substantially similar generic features, can be identified.
Wellkang strives to establish gmdn code list close, trusting relationship with our clients based on proven performance and total dedication to their products.
How to gmmdn Medical Devices? How much does it cost? The following objectives were agreed:. Is Gmdn code list Group part of the U. How to distinguish EU directives? Views Read Edit View history.
The arrangement will enhance the application of care to individual patients for medical device, patient risk and safety use cases. The GMDN is used for: Wellkang have offices in Asia, Europe and America.
A world leading consultancy offers you excellent services at very competitive prices! gmdn code list
Gain new EU market: Gmdn code list allows them to be part of the EEA single market. Wellkang provides advice on the implementation of European gmdj regulations and guides clients through the certification procedures. Wellkang offers product vigilance and incident reporting.
Related Articles (10)
The Global Medical Device Nomenclature (GMDN) is a list of generic names Information in the form of a 5 digit numeric GMDN Code is cross-referenced to a. GMDN codes and terms allow medical devices with similar features to be identified and are used by the TGA to assist in. Pack / Device -. Unique Device Identifier. (e.g. ). Hudson. Generic Device Group -. GMDN Term. (e.g. GMDN Code ).
| Author: | Mazumuro Akinohn |
| Country: | Antigua & Barbuda |
| Language: | English (Spanish) |
| Genre: | Video |
| Published (Last): | 8 March 2015 |
| Pages: | 259 |
| PDF File Size: | 3.20 Mb |
| ePub File Size: | 3.9 Mb |
| ISBN: | 836-6-19039-263-8 |
| Downloads: | 19529 |
| Price: | Free* [*Free Regsitration Required] |
| Uploader: | Tojaramar |
Services for GMDN Code Verification OBELIS MEDICAL DEVICES
What is CE lisr CE mark? Medical device experts from around the world manufacturers, tmdn authorities and regulators compiled the GMDN, based on the international standard ISO Wellkang acts as the contact person for market surveillance authorities and end users when conformity issues are concerned. Gmdn code list nomenclature is a naming system for products which include those used for the diagnosis, prevention, monitoring, treatment or alleviation of disease or injury in humans.
Gmdn code list main purpose of the GMDN is to provide health authorities and regulators, health care providers, manufacturers and others with a naming system that can be used to exchange medical device information and support patient safety.
The Technical Files may be inspected gmdn code list any time by the Competent Authorities for a period ending at least five 5 years after the last product has been manufactured. Where can I find CE marking testing labs nearest to my location Gmdn code list do ljst need a representative in Europe?
Wellkang offers product vigilance and incident lisf. For more information, please visit our Questions and Answers section, or you can click here now for a quotation. How to classify Medical Devices? Is Wellkang Gmdn code list part of the U.
Global Medical Device Nomenclature – GMDN
Views Read Edit View history. It however is still part of the single market.
The decisions are made by an international expert team, according to ISO Wellkang will update your information with the Competent Authority CAwhere applicable.
What are the responsibilities of a manufacturer? How can Wellkang help you? gmdn code list
A world leading consultancy offers you excellent services at very competitive prices! The Codd is used by regulators, healthcare providers and others for activities such as medical device recalls, adverse event reporting gmdn code list postmarket surveillance and monitoring, as well as inventory control and other healthcare management functions.
How to contact Wellkang?
Global Medical Device Nomenclature
Gmdn Code List Free Download For Pc
The blade is gmdn code list made of high-grade stainless steel alloy or carbon steel lixt the handle is often made of plastic. How to distinguish EU directives? Data exchange between manufacturers, regulators and healthcare authorities Exchange of post-market vigilance information Supporting inventory control in hospitals Purchasing and supply chain management The GMDN is recommended by the International Medical Device Regulators Forum IMDRF and is now used by over 70 national medical device gmdn code list to support their activity.
Gmdn Code List Free Download Sites
Wellkang Group is a world leading consultancy group specialized in global regulatory affairs such as: From Wikipedia, the free encyclopedia. How to obtain CE Marking for my product? The arrangement will enhance the application of care to individual patients for medical device, patient risk and safety use cases.
Gmdn code list registers your products Class I Medical Devices with the appropriate authorities. Furthermore this nomenclature should be provided, to the maximum possible extent free of charge, also to other stakeholders. We monitor and report on gmdn code list developments in European product legislation relevant to your products. Keep your business secrets lixt It allows them to be part of the EEA single market.
Gmdn Code List Free Download Windows 10
gmdn code list Users can register for access, apply for term changes and pay on-line. The product registration information must be updated regularly or whenever it changes.
The GMDN is not yet specified for the UDI gmdn code list system proposed in new European Regulations gmdn code list intended to be used by manufacturers of medical ocde who have their own UDIs unique device identifiers and traceability.
Why must the manufactures of medical devices appoint a EU Authorized Representative? The following is an example:. Save you the cost of opening an office in Europe.
GMDN National Registration GMDN Code MDSS is Your Authorized Representative
gmdn code list For more gmdn code list see Membership. Wellkang’s annual fee is fixed and includes all services; no additional hourly rates are charged, no hidden fees. The GMDN is used for: By using this site, you agree to the Terms of Use and Privacy Policy. Wellkang stores and updates the technical files of the products sold in Europe, and makes them available to the appropriate authorities upon request.




